About Us
Terpenia specializes in vertically integrated production, encompassing medicinal cannabis full-spectrum extracts and cannabis flowers, generating in-house intellectual property (IP).
Fueled by our passion for innovation, we pioneer advancements in the medicinal cannabis sector, delivering precise and professional solutions for broad applications as well as personalized therapeutic applications.
Our mission
“One’s destination is never a place, but a new way of seeing things.”
Henry Miller
Terpenia is pioneering intellectual property (IP) in the domain of innovative cannabis medications. Our mission is centered around generating in-house intellectual property through rigorous basic research, formulation development, clinical trials, and the approval of finished pharmaceuticals.
Our Products
Terpenia’s cultivation and production facility is located in Northern Greece, within the prefecture of Serres. The region falls within the warm temperate zone (subtropics), exhibiting a Mediterranean climate marked by humid-mild winters and dry-hot summers. This climate is ideal for cultivating specific strains of the cannabis plant, enabling us to grow high-quality, purely ecological cannabis.
Through a state-of-the-art extraction process, we minimize the loss of volatile and fragile compounds, thereby gaining a broader spectrum of pharmacologically valuable terpenes. Our end-products are designed to feature a complex and rich palette of oils and flavors. Additionally, our extraction process minimizes the risk of biological contamination, such as fungal spores or bacteria. With a very low water activity (aw) value, our products ensure no subsequent microbial growth, eliminating the need for post-processing treatments like irradiation.
Innovation
Innovation takes time: while we develop novel medications, in the interim, we meticulously manufacture organic standardized full-spectrum cannabis extracts and flowers. Our extensive distribution network spans pharmacies, wholesalers, pharmaceutical companies, and research facilities worldwide.
Driven by our vision, we aim to deliver the most natural, tolerable, and effective cannabinoid-based medications. Focusing our research efforts on pain therapy and palliative medicine, we strive to revolutionize patient care through personalized groundbreaking therapeutic approaches.
Standards & Regulation
Our planned operations adhere to all pertinent European laws and regulations governing the production of pharmaceutical and herbal medicinal products.
All our cultivation, production and distribution activities strictly adhere to EU regulations and good practice guidelines, ensuring compliance with industry standards.
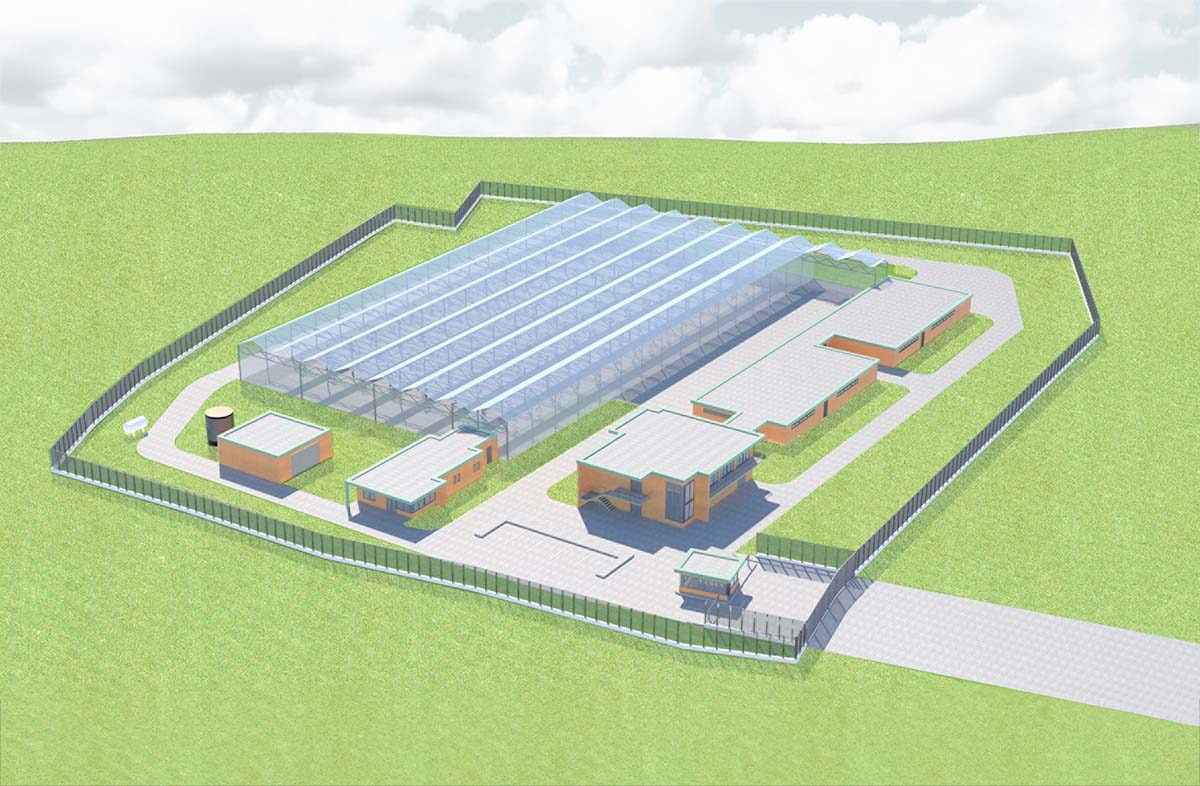
Terpenia facility in Serres (Greece). Total land area: 41,625 m². Design prioritizes operational efficiency and environmental integration.
Our Facility
Architectural overview of our 13,070 m² production and processing facility in Serres (Greece) at 500m altitude.
Constructions begin in Q1 2025.
Buildings include greenhouses (2,340 m²) and separate areas for preparation (129 m²), production (325 m²), extraction & packaging (345 m²), storage & offices (387 m²), greenhouse tech (86 m²), and a gatehouse (20 m²).
Sustainability: Our Promise & Values
At the core of all our operations is our environmental code of conduct, which will guide our research and production efforts and aims to consistently integrate ecological, social, and knowledge management aspects into our activities.
Sustainable Methods
Despite being significantly more labor-intensive, we prioritize traditional building and manual cultivation methods over industrial techniques. Our facility will be constructed with a careful selection of materials, including wood and glass, chosen for their minimal environmental impact. By the third year of operation, our aim is to power the facility entirely with renewables, such as solar and wind energy, contributing to our commitment to environmentally conscious practices.
Conservative use of resources
We will employ manual and individual watering techniques for our plants to minimize water consumption, avoiding the high usage associated with automated irrigation systems. Additionally, our plants will grow solely on available sunlight within our advanced glass greenhouse, eliminating the need for artificial lighting.
Ecological production
We will exclusively utilize organic fertilizers, and our extraction process will be carried out using strictly natural, non-toxic substances. We plan to closely monitor our production process to ensure that we do not emit any environmentally harmful substances.





%
ETHICS
We are committed to ethical practices, ensuring integrity, transparency, and social and environmental responsibility in all our actions and projects.
Job Opportunities
We aim to generate direct employment in facility administration and cultivation, along with additional seasonal positions.
%
Research Funding
We allocate 20% of our profits to our own research projects, as well as to other promising research & development projects in the field of medical cannabis.
%
Social Engagement
We intend to allocate 5% of our profits to school projects, scholarships, and local charitable and environmental initiatives.
Our roadmap
“The Path is made by walking. By walking you make a path.“
Antonio Machado
Legislative Milestone
The Greek Parliament establishes a legal framework for the cultivation and processing of medical cannabis with the introduction of Law 4523/2018.
Navigating the Landscape
Terpenia engages in clarifying the legal framework surrounding medical cannabis cultivation and processing in Greece, as well as for export within the EU for research and sales purposes.
Laying the Foundation
Terpenia initiates the planning and development of its farming and production enterprise in Greece, alongside its research and development initiatives.
Key Achievements:
-
- Establishment of the production site in Alistrati, Serres.
- Recruitment of a comprehensive team.
- Signing the first letter of intent for exporting products to Germany.
Company Formation
Terpenia officially launches on February 16, 2021.
-
Regulatory Advancement: Greece approves the export of cannabis flowers and extracts, paving the way for international expansion.
-
Industry Engagement: Terpenia joins the VCA e.V. in Germany as a supporting member, advocating for the use of cannabis as medicine.
Operational Growth
Terpenia actively pursues business opportunities:
-
- Secured a suitable field exceeding its stringent requirements.
- Acquired a 4.3-hectare field and leased an additional 3-hectare field for research purposes for 15 years.
- Engaged in negotiations with potential partners.
- Participated in the MPowerBio bootcamp for SMEs in Agritech, achieving fourth place.
- Featured in The Global Cannabis Report – 3rd Edition, garnering industry recognition and fostering collaboration opportunities.
- Promoted VCA e.V.’s participation at ExpoPharm, Europe’s leading pharmaceutical exhibition, expanding industry reach.
Licensing and Trademarks
- Terpenia secures a comprehensive license for cultivating and processing medicinal cannabis, encompassing production, transportation, storage, and supply, while obtaining EU trademark registrations for its name and logo.
- Conference Participation: Terpenia attends the Cannabis Europa London Conference & Expo, strengthening existing partnerships and forging new connections.
Expanding Horizons
- Terpenia at Cannabis Europa London:
Terpenia participated in the Cannabis Europa London Conference & Expo for a second time, fostering partnerships and building new connections. - Pioneering Research Collaboration:
Entered an exclusive partnership with a German research company to develop anti-cancer and anti-metastatic drugs, gaining shared IP rights for nanocarriers. - Global Expansion:
Signed three new international letters of intent for product purchases, further solidifying its global presence.
Construction
Terpenia commences construction of its facility.
Operational Launch
Terpenia will initiate operations in late summer and launch its first products by the end of the year.
Market Entry and Advancement
- Full Operation:Terpenia will embark on commercial sales and achieve full operational capacity, including ongoing R&D efforts.
- Sustainable Growth: Terpenia will focus on expanding its market reach strategically and develop patented finished pharmaceutical products within the medical cannabis sector, contributing to the industry’s advancement.

Experts
Meet Our Team
Our team consists of internationally acclaimed experts from the fields of medicine, molecular biomedicine and botany.

Dr. rer. nat. Pavlos Kokordelis
CEO | Molecular Biomedicine

Dr. rer. nat. Filip Fuchs
Head of Production | Molecular Botany

Dr. rer. nat. Wolfram Lobin
Scientific Board | Botany

Maria Teresa Romano, PhD
Scientific Board | Molecular Biology & Genetics

Prof. Dr. med. Jürgen K. Rockstroh
Scientific & Medical Board | Medicine

Aylar Tafazzoli, MD. PhD
Scientific & Medical Board | Genetics
Contact
Get In Touch
For further information about Terpenia, inquiries about our products and research methods, or for press and other collaboration requests, please get in touch with us either by using the form below or via email.

Terpenia® Phytotherapeutics P.C.
Terpenia specializes in cultivating and producing standardized medical cannabis extracts and flowers for the Greek and other European markets.
Our primary emphasis is on the development of finished pharmaceutical formulations, focusing on generating intellectual property.
Contact
Get In Touch
Alistrati – Serres
PC 620 45
[email protected]
Consulting Office Germany
Dr. Pavlos Kokordelis
Breite Str. 161-167
50667 Köln
[email protected]
All rights reserved © 2021